Abstract
Background and Scientific Rationale
Lenalidomide maintenance after autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma (MM) patients has demonstrated an improved progression free survival (PFS) and overall survival (OS) when compared to placebo or observation in a meta-analysis (McCarthy et al. JCO 2017). Despite these improvements, patients on maintenance had higher rates of second primary malignancy and discontinuation due to treatment-emergent adverse events which may lead to decreased quality of life (QoL). Most MM patients are treated with lenalidomide maintenance in the absence of great alternatives. Thus, there is an unmet clinical need to develop alternative maintenance strategies.
Daratumumab is well tolerated and effective in MM, with many studies continuing daratumumab as maintenance until disease progression. The proposed study seeks to further investigate and directly compare patient reported QoL between maintenance lenalidomide and daratumumab.
Study Design and Methods
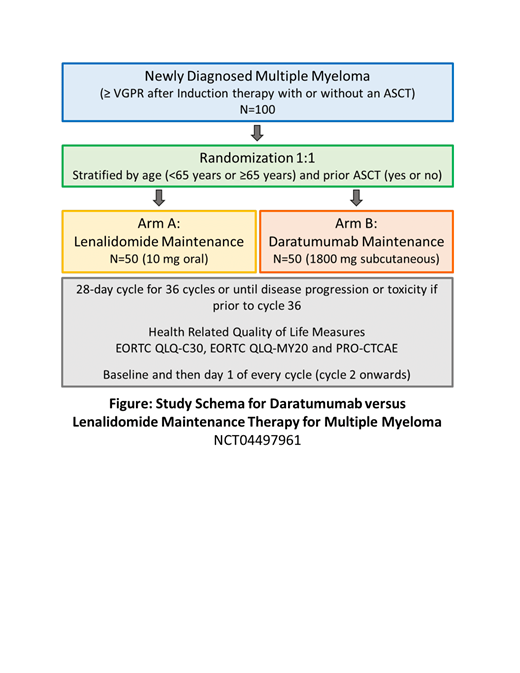
This is an open-label single-center randomized pilot study of daratumumab versus lenalidomide maintenance in 100 newly diagnosed MM patients (Figure).
Clinical trial registry number: NCT04497961, actively recruiting.
Study population
A total of 100 patients will be enrolled, randomized 1:1 to each arm (50 patients per arm). Randomization for enrollment will be stratified by patient age (<65 years and ≥65 years) and prior ASCT status (yes or no).
Inclusion criteria
Newly diagnosed MM treated with combination therapy with or without ASCT
Documentation of a very good partial response or better.
Enrollment within 6 months of completing initial combination therapy.
Enrollment following minimum 100-day washout per standard guidelines in ASCT recipients.
ECOG performance status ≤ 2.
Exclusion criteria
Progressive or refractory MM.
History of disease refractory to lenalidomide or daratumumab.
History of prior anti-myeloma therapy for smoldering MM.
Currently receiving other investigational agents to treat MM.
Study treatment
The length of therapy on both arms is 36 cycles of 28 days (~3 years), or until disease progression or unacceptable toxicity.
Subjects under the lenalidomide arm will be treated with a maintenance dose of lenalidomide 10mg per day on days 1 to 21 of each cycle. Subjects under the daratumumab arm will be treated with a standard subcutaneous dose of 1800mg weekly in cycles 1 & 2, followed by every 2 weeks in cycles 3-6 and every 4 weeks cycles 7-36.
Study assessments
Subjects will complete three validated questionnaires - EORTC QLQ-C30 (general QOL in cancer patients), EORTC QLQ-MY20 (QOL in MM patients) and PRO-CTCAE (adverse events). These will be collected at baseline, day 1 of cycle 2, every cycle day 1 thereafter, at study/therapy discontinuation, and at 1-month post therapy follow-up. The GHS score will be calculated based on questions from the EORTC QLQ-C30.
Endpoints
Primary
To compare differences in GHS scores between patients receiving lenalidomide versus daratumumab maintenance
Secondary
To explore differences in EORTC QLQ-C30, EORTC QLQ-MY20 and PRO-CTCAE scores
To compare CTCAE adverse events
To evaluate differences in PFS and OS
To estimate differences in minimal residual disease status
To explore the association between minimal residual disease status and clinical outcomes
Exploratory
To compare minimal residual disease techniques of multi-parametric flow with next-generation sequencing and mass spectrometry
To assess differences in T cell, NKT, NK cell subtypes
To explore associations between disease biology and clinical outcomes using genomic sequencing.
To assess changes in the stool microbiome
Statistical methods
This protocol is a randomized pilot study to estimate the difference in the global health status for patients receiving lenalidomide maintenance to patients receiving daratumumab maintenance. The primary endpoint is the GHS from EORTC QLQ-C30. The primary analysis will be performed using a linear mixed effects model. The randomization stratification factors will be included as covariates in the regression model. The primary endpoint evaluation will use all GHS available up until the final evaluation at 36 cycles.
Shah: Celgene/BMS: Research Funding; Janssen: Research Funding. Mailankody: Bristol Myers Squibb/Juno: Research Funding; Fate Therapeutics: Research Funding; Physician Education Resource: Honoraria; Evicore: Consultancy; Legend Biotech: Consultancy; Plexus Communications: Honoraria; Jansen Oncology: Research Funding; Allogene Therapeutics: Research Funding; Takeda Oncology: Research Funding. Korde: Medimmune: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding. Hultcrantz: Intellisphere LLC: Consultancy; Daiichi Sankyo: Research Funding; Amgen: Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Research Funding; Curio Science LLC: Consultancy. Hassoun: Celgene, Takeda, Janssen: Research Funding. Scordo: Angiocrine Bioscience: Consultancy, Research Funding; Omeros Corporation: Consultancy; Kite - A Gilead Company: Membership on an entity's Board of Directors or advisory committees; i3 Health: Other: Speaker; McKinsey & Company: Consultancy. Dahi: Kite / Gilead: Membership on an entity's Board of Directors or advisory committees. Lahoud: MorphoSys: Membership on an entity's Board of Directors or advisory committees. Landau: Takeda, Janssen, Caelum Biosciences, Celgene, Pfizer, Genzyme: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Genzyme: Honoraria. Shah: Janssen: Research Funding; Amgen: Research Funding. Giralt: Actinnum: Membership on an entity's Board of Directors or advisory committees; SANOFI: Membership on an entity's Board of Directors or advisory committees; AMGEN: Membership on an entity's Board of Directors or advisory committees; CELGENE: Membership on an entity's Board of Directors or advisory committees; PFIZER: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; JENSENN: Membership on an entity's Board of Directors or advisory committees; JAZZ: Membership on an entity's Board of Directors or advisory committees. Lesokhin: Behringer Ingelheim: Honoraria; bristol myers squibb: Research Funding; pfizer: Consultancy, Research Funding; Serametrix, Inc: Patents & Royalties; Trillium Therapeutics: Consultancy; Genetech: Research Funding; Iteos: Consultancy; Janssen: Honoraria, Research Funding. Landgren: Amgen: Honoraria; Janssen: Other: IDMC; Amgen: Research Funding; Janssen: Research Funding; Janssen: Honoraria; Celgene: Research Funding; Takeda: Other: IDMC; GSK: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal